1 Introduction
Magnesium Stearate [MS] (CAS: 557-04-0) is a common pharmaceutical excipient. MS is a white, fine powder with fluffy texture, low bulk density, poor fluidity, and soft to the touch. It has a slight odor and is insoluble in water, ethanol and ether. Magnesium is compounded with stearic acid. Calculated as dry product, the magnesium content should be 4%-5%.
Magnesium stearate is often used as a lubricant in the production of capsules and tablets, which can reduce the friction between particles and between the contact surfaces of the particles and the manufacturing equipment (such as tablet punches and dies). An important variable in lubricating performance is the specific surface area. The larger the specific surface area, the easier it is to distribute evenly during the mixing process.
Magnesium stearate can transition into an amorphous state or crystalline hydrate after absorbing water. Hydrate includes: monohydrate, dihydrate and trihydrate. Darren P et al. [1] pointed out that during the heating process, magnesium stearate’s free water is removed below 40°C, bound water is removed between 70-105°C, and MS is melted above 110°C.
The 2020, several international pharmacopoeias stipulated that magnesium stearate should be marked with a specific surface area value, and the US Pharmacopeia stipulated that the degassing condition of magnesium stearate should be 40°C under vacuum for 2 hours [2]. This article aims to explore the precautions and procedures for magnesium stearate in the specific surface area test process.
2 Specific surface area test program
Test sample: Magnesium stearate;
Test instrument: AMI-Meso 112 specific surface area and pore size analyzer
Fig. 1 photo of AMI-Meso 112
2.1 Pretreatment and general requirements
a. Sampling amount:
The US Pharmacopeia stipulates that nitrogen is used as adsorbate, and the area to be tested is > 1m2.
b. Gas:
High-purity nitrogen, purity>99.999%;
High purity helium.
c. Pretreatment:
Before the test, the magnesium stearate sample was degassed by heating under vacuum.
Magnesium stearate is light in weight, fluffy, and has a low bulk density. When pumping a vacuum, you need to pay attention to prevent it from dislodging. In the vacuum option of AMI-PAS software, the vacuum pumping speed is divided into "Fine Powder", "Powder" and "Particles" according to the material type. For the magnesium stearate samples, select "Fine Powder" to avoid any sample being pulled into the vacuum system.
2.2 Sample test
The steps of the static volume method to test specific surface area are as follows:
a. Weigh the sample: use a one-tenths balance, first weigh the mass of the empty tube, record mass1, then weigh an appropriate amount of sample, pour it into the sample tube, record the sample + the mass of the empty tube mass2; (Note: Weigh 1.2g in this experiment)
b. Pretreatment: Put the sample tube in the pretreatment position or degasser for degassing, set the degassing temperature and degassing time (Note: the degassing condition of this experiment is 40C for 2h);
c. Review mass: After the pretreatment is completed, the weight the sample again and record it as mass3;
d. Start experiment: put the sample tube in the test position, set the test conditions, put the Dewar flask filled with liquid nitrogen in the tray corresponding to the test position, and click to start the experiment;
e. After the experiment is completed, export the report.
2.3 Results for a specific sample
BET specific surface area test data
Single-point BET specific surface area at p/p0 0.20000: 3.99427 m2/g
BET specific surface area: 4.94369 m2/g
Slope: 0.82547
Intercept: 0.05496 cm3/g STP
Monolayer saturated adsorption capacity Vm: 1.13580 cm3/g STP
C value: 16.01841
Linear factor Cc: 0.99985
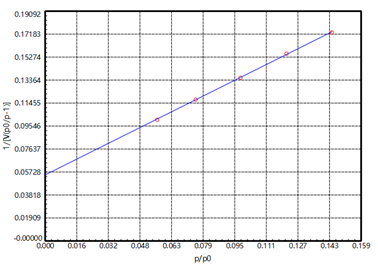
Figure 2
The United States Pharmacopoeia stipulates that the specific surface area of magnesium stearate should be determined in the range from P/P0 0.05-0.15, and the linear factor of the specific surface is Cc>0.9975. It can be seen from [Figure 2] that in the BET test result of magnesium stearate, the linear factor Cc satisfies >0.999. C>0, which means that the BET test result is reliable.
3 Conclusion
Guidelines for testing the specific surface area and pore diameter of magnesium stearate:
(1) Magnesium stearate is light, fluffy, and has a low bulk density. Use "AMI-PAS" software, select the "Fine Powder" vacuum option.
(2) Use nitrogen (purity> 99.999%) as the adsorbate, and the area to be measured is at least 1m2.
(3) The specific surface area selection range of magnesium stearate is P/P0: 0.05-0.15, and the linear factor of the specific surface area should be greater than 0.9975.
The AMI-Meso series specific surface area and pore size analyzer is a fast and economical instrument that can meet multiple pharmaceutical test methods. This instrument can not only reliably test ultra-low specific surface area samples, but it can also help determine product performance and even help predict the promise of a specific sample’s application. In addition, rapid determination of specific surface area and other parameters can easily enable manufacturers to focus on quality control in order to yield better a better pharmaceutical product.
Reference
[1]Darren P. Lapham, Julie L. Lapham. Gas adsorption on commercial magnesium stearate: Effects of degassing conditions on nitrogen BET surface area and isotherm characteristics[J]. International Journal of Pharmaceutics, 2017,530:364-376.
[2]The United States Pharmacopeia. Magnesium Stearate, 2012:1850.





